We are developing products concentrating on “regenerative medicine and cell therapy.”
VICX Therapeutics aims to provide regenerative drug products as a part of the most advanced medical technology available.
The safety and benefit of regenerative medicines for treating various types of diseases has been demonstrated in clinical studies . More importantly, side effects and the onset of chronic complications associated with regenerative medicines are limited, which has a very significant meaning for considering the extension of a healthy life expectancy; since a patient can be expected to recover his/her health while maintaining a good QOL (quality of life). Although some regenerative medicines are now commercialized as a clinical service, regenerative medicines as approved medical drugs in Japan is highly expected to occur in the near future.
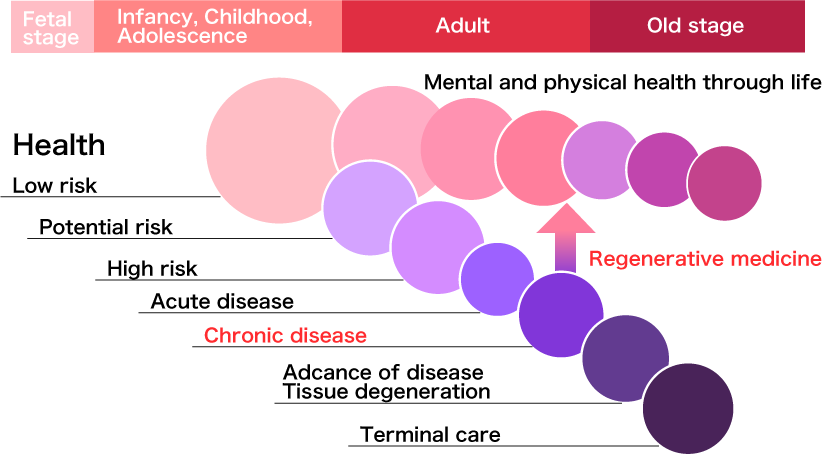
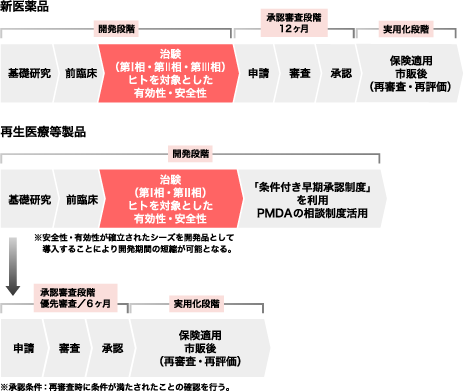
The market scale of regenerative and cell therapies in Japan is forecasted to be \95 billion, \1 trillion and \2.5 trillion in 2020, 2030 and 2050, respectively. For these reasons, academic and research institutes, as well as members of the medical industry, are successfully obtaining the potential seed products for commercialization. Furthermore, the Revised Japanese Pharmaceutical Law gives us an opportunity to provide patients with new regenerative drug products following a short development period through obtaining conditional approval for their practical use in patients. The differences between the former and revised pharmaceutical laws in Japan are indicated in the flow chart on right side.
Since it is generally assumed that the characteristics of cell/tissue-processed drug products tend to be inconsistent in manufacturing and quality and may differ in their efficacy within each individual, regenerative drug products can now be approved in Japan under the new approval system by presumption of their efficacy in the clinical trial. Thus, under the new approval system the quick practical use of an advanced therapy in patients can be expected. However, efficacy of these products will be a major regulatory inspection issue for the final approval process of regenerative drug products during the post-marketing period following from their conditional approval. Therefore, the importance of safety and efficacy remains the same as it did under the former law. To avoid a case where a regenerative product with conditional approval would fail to be qualified for final approval after the verification period, and patients are accordingly forced to stop using the product, it is imperative that we select promising seed products that are truly ready for commercial development.
Therefore, VICX Therapeutics aims to introduce promising therapies selected from seed products with proven safety and efficacy that has been established in clinical studies conducted in either Japan or overseas, or through in-licensing of approved drug products currently only available in overseas markets.
Business Activities
- Development of drug products relating to regenerative and cell therapeutics
- Planning, development and application for approvals of possible drug products.
- Translation and sales of various rights of investigational new drugs and approved drugs.
Features (Novelty, Innovation and Growth)
- Novelty
- We sell regenerative drug products derived and processed from human cells and tissues.
- Innovation
- We utilize the functions of cells and tissues for medical therapies.
- Growth
- The drastic growth of this particular industry is expected in the future pharmaceutical/medical market. Japan is already facing a rapidly aging society in which the population of these patients is increasing year by year; thus, the demand for innovative medications will rise. The number of users is expected to grow in accordance with the expansion of target diseases.
Development process
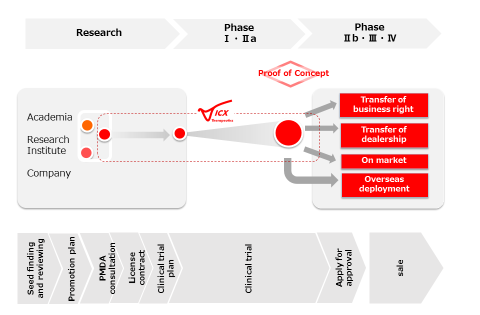
We will progress the development of a promising treatment, who’s translational clinical study(s) was conducted at a university or research institution in either Japan or foreign countries. As yet unapproved in Japan, commercialization of promising products with demonstrated safety and efficacy, as cutting-edge medicines and treatments will be developed for regulatory approval in Japanfollowing the process shown in the left figure and ① ~ ⑤ below.
①Excavate a promising seed therapy that is buried in a university or research institute.
②Review its development possibility in accordance with PMDA consultations.
③Introduce it into the development path by an appropriate scheme (e.g., in-license, joint company/collaboration).
④Conduct clinical trials to evaluate the safety and efficacy of the seed product.
⑤Derive it for sale according to its features after approval.
Development period
The preparation period to enter into a clinical trial can be shorten by introducing a promising seed product which has established safety and efficacy. The clinical trial period can also be shorten by pursuing the conditional approval path of the current revised pharmaceutical law. Therefore, we can design a business model aiming to obtain conditional approval to start sale of the drug product in a short period.
Pipelines
We are planning to develop regenerative therapy using somatic stem cells and immuno-cell therapy using immune cells.
Regenerative therapy
Somatic stem cells exist in the bone marrow, adipose tissues and placenta, and have the capability to promote repairing of an inflammation tissue site by anti-inflammation effects and secretion of growth factors. Regenerative therapy is aimed to improve the function of cells in a lesion site by administration of somatic stem cells. The somatic stem cells isolated from a tissue are expanded or differentiated ex vivo for clinical use and are administrated into a lesion site of the patient. Some of these regenerative therapies, including those targeting cartilage and skin, are already commercialized.
Immuno-cell therapy
When the immune system can no longer control cancer cells, which emerges in the cancer patient’s body for variety of reasons, cancer cells can inexhaustibly proliferate. Immuno-cell therapy is aimed to re-control the proliferation of cancer cells by “artificially” eliciting the immune response against cancer cells ex vivo. Immune cells derived from the patient’s peripheral blood are isolated and cultured for their activation. The activated immune cells are then administrated into the patient for successful cancer treatment therapy.